
They examined the safety outcomes as well, including adverse events and clinical lab assessments through the last patient visit. The investigators measured efficacy using clinical and microbiological responses at a test-of-cure visit, which was timed according to the protocols of each of the 5 studies.
#Antibiotics to treat gram negative rods plus#
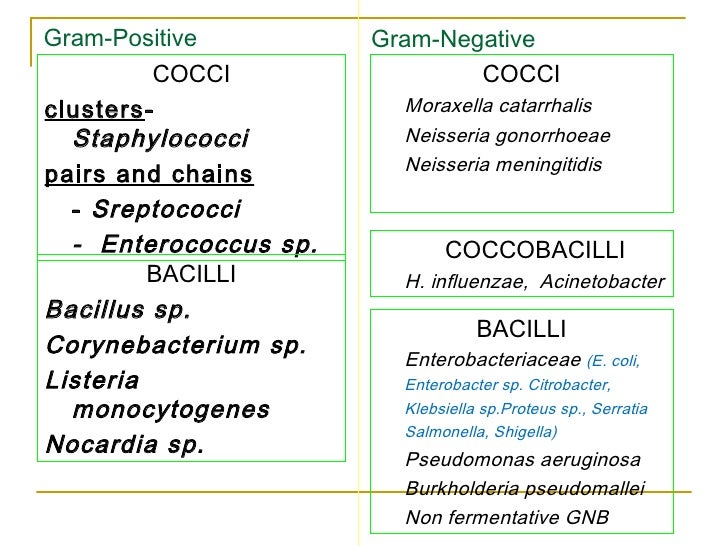
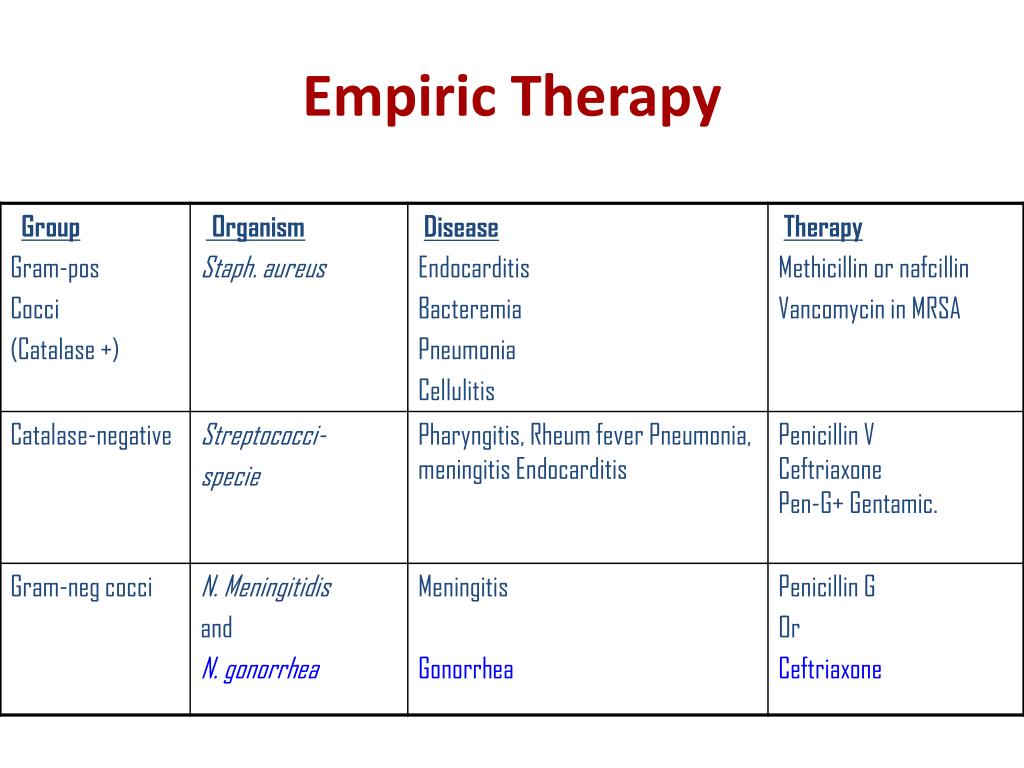
In a majority of the studies, the comparators were carbapenems the patients with cIAI were treated with ceftazidime-avibactam plus metronidazole. The treatment durations varied in each of the studies. In each of the trials the study authors reviewed, patients were randomized to receive either intravenous ceftazidime-avibactam or comparators for between 5-21 days. The agency believes their “aggressive recommendations” can prevent the spread of gram-negatives. Additionally, they can pass along genetic material that can allow other bacteria to also become resistant to drugs. Investigators from the US, Germany, Spain, and the UK reviewed 5 randomized, controlled, multi-center phase 3 trials in adults with complicated intra-abdominal infection (cIAI), complicated urinary tract infection (cUTI), hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) - all infections caused by ceftazidime non-susceptible and multidrug-resistant gram-negative bacteria.Īccording to the US Centers for Disease Control and Prevention (CDC), Gram-negative bacteria can cause infections, are resistant to multiple drugs, and are increasingly resistant to most available antibiotics. Ceftazidime-avibactam was safe and effective for patients with a variety of gram-negative bacterial infections, according to an abstract planned for presentation at the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) 2020 Meeting this year.


 0 kommentar(er)
0 kommentar(er)
